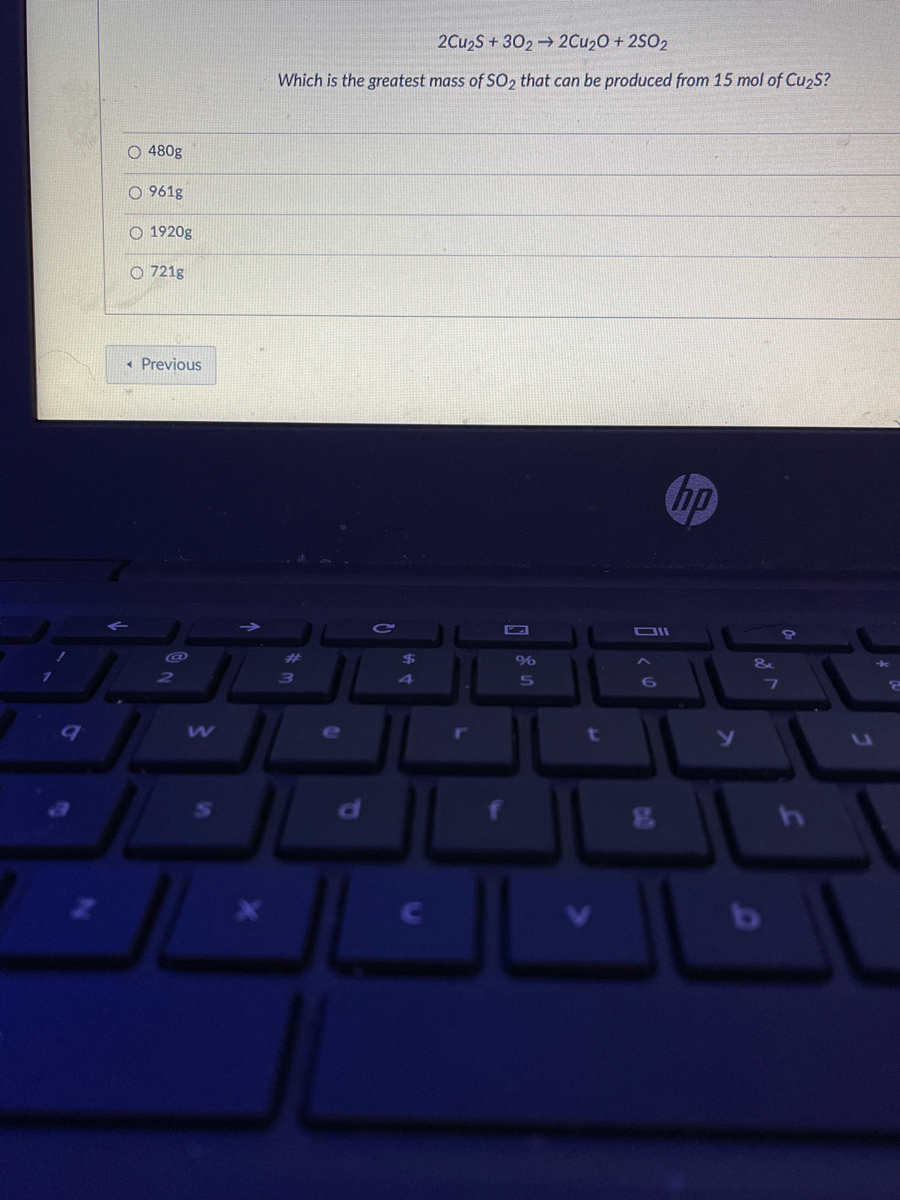
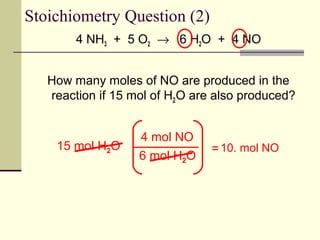
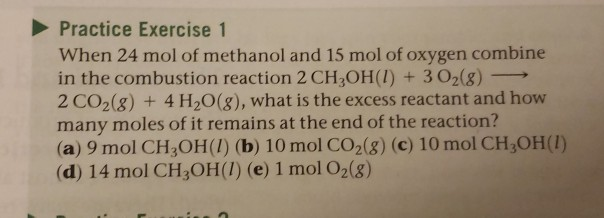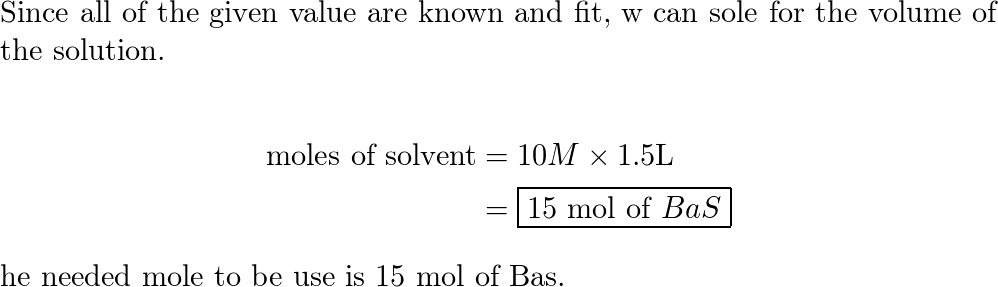A reaction vessel contains initially contains 4.0 mol of NO and 15.0 mol of H2. How many moles of NO, H2, NH3 and H2O are there inside the reaction vessel once the

りん酸緩衝剤粉末 (1/15 mol/L pH 7.4)・Phosphate Buffer Powder (1/15 mol/L, pH 7.4)・167-14491【詳細情報】|【ライフサイエンス】|試薬-富士フイルム和光純薬

XRD pattern of (a) pure PVP and PVP doped with (b) 10 mol% (c) 15 mol%... | Download Scientific Diagram

15 moles of H2 and 5.2 moles of I2 are mixed and allowed to attain equilibrium at 500 ^o C. At equilibrium, the concentration of HI is found to be 10 moles.
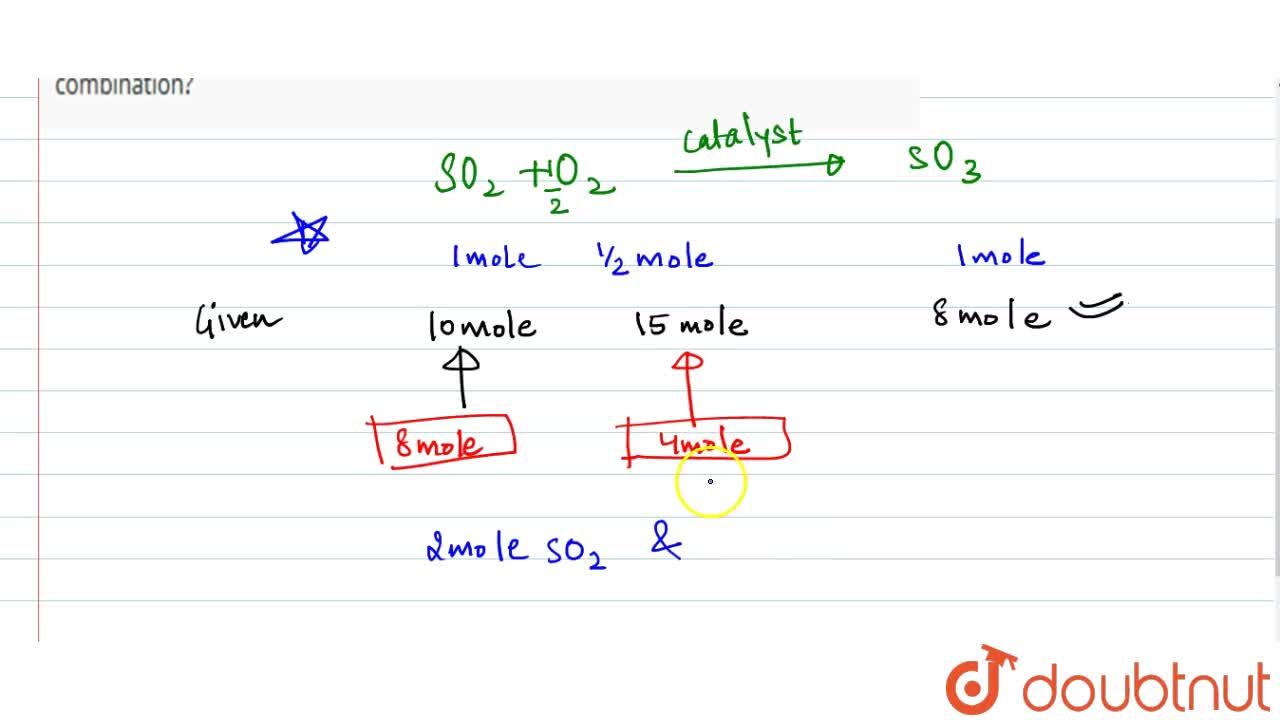
When a mixture of 10 moles of SO(2) and 15 moles of O(2) was passed over catalyst, 8 moles of SO(3) was formed. How many moles of SO(2) and O(2) did not

15 moles of N2 is mixed with 20 moles of H2 in an 8 litre vessel. 5.6 moles of ammonia is formed Calculate Kc for the equation, N2(g) +3 H2(g)= 2NH3(g) "+ heat"

For, A + B C , the equilibrium concentration of A and B at a temperature are 15 mol litre^-1 . When volume is doubted the reaction has equilibrium concentration of A

Nickel‐Catalyzed Thiolation of Aryl Nitriles - Delcaillau - 2021 - Chemistry – A European Journal - Wiley Online Library